Soil chemistry is often one of those things you don’t think too deeply about until you encounter a problem that you can’t solve and are forced to do some in-depth research. Why do your crops keep under-performing in spite of beautiful looking soil? Why do some types of plants thrive while others barely survive?
The principles of soil chemistry therefore often fall into the category of ‘things you didn’t know that you didn’t know,” but the best way to start investigating your soil chemistry is to get a soil test. We’ve talked before about some basics, and how to read a bit deeper into the N,P and K levels in your fertilizer. This time, we’ll focus on some essentials of soil chemistry that are often hidden in your basic soil tests.

Key Features of a Soil Test:
- Cation Exchange Capacity
- Organic Matter Content
- pH
- Nutrient Levels
- Nutrient Balance
Soil Chemistry: Cation Exchange Capacity
One of the main concepts of soil chemistry is the cation exchange capacity of your soil. Cations are positively charged elements, including important ones for you soil such as calcium, magnesium, and sodium. The cation exchange capacity is the amount positive charge that your soil can absorb – rather, the ability of negatively charged elements in your soil that can hold on to positively charged chemicals – or cations. It’s helpful to think of this as the ‘gas tank that can hold soil fertility’.
Your ideal cation exchange capacity, or CEC, is between 10 – 30 mg per 100 grams. In really sandy or really abused soils, it can be as low as 3 or 4 percent. A soil with a low CEC can’t hold onto much fertility. Unfortunately, no matter what you do, this is pretty well set in stone and you can only change this number a little bit. Methods to help address this include adjusting your soil pH and/or increasing organic matter in your soil (something I recommend across the board as it is).
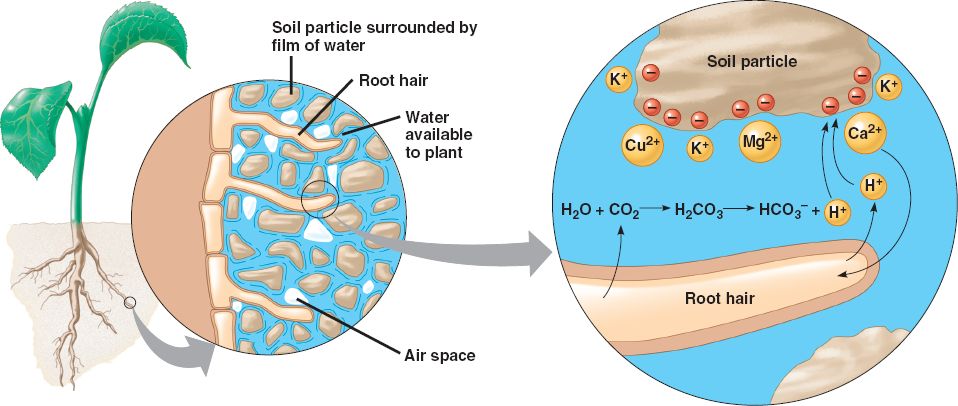
So, while you can’t really change your CEC, knowing what yours is and understanding what that means can help you and your garden. For one, it will help you determine how best to deliver nutrients to your soil. Soils with a low CEC will respond really well to liquid fertilizers, for example, because they are quickly absorbed and utilized.
Soil Chemistry: Organic Matter
We talk a lot about organic matter content in soils. Try as you might, the reading will only change slightly during your lifetime. I’m happy to report that at Petaluma Bounty our numbers have slowly crept up every year – from 3.1%, to 3.2%, and now up to 3.4% organic matter. I jumped for joy! As all the readers know, one great way to add organic matter to your soil is to make compost for the garden. But you’re all already doing that, right?
Soil Chemistry: pH
This is one of the more changeable elements of your soil, and one that is fairly well understood by many resources out there including agricultural extension agents and master gardeners. The ideal pH range for many crops is between 6.5 – 7. When soils are either on the very high or very low end of this spectrum, a range of issues can occur. One common issue on farms is the inability for soil with a high pH to take up iron, therefore inhibiting plant growth.

Many home gardeners are aware of soil pH as it relates to finicky crops that have very specific pH needs. Blueberries, hydrangeas, and rhododendrons come to mind as crop that many of us grow that need highly acidic soil. At the Bounty, we’ve been adding apple cider vinegar to our soil to help our blueberries thrive with the added acidity.
Soil Chemistry: Percent Base Saturation
This concept took me a little while to understand. Remember those cation exchange sites? Well the percent saturation is the proportion of these sites that are occupied for the nutrients we’re looking for – the ‘base’ refers to the fact that the number will refer to a standardized group of commonly measured nutrients. Understanding this number is a great thing – it can help you to predict your soil’s ability to feed and support your crops.
Soil Chemistry: Nutrient Levels and Nutrient Balance
Going with the theme of learning the things we didn’t know that we didn’t know, it’s worth taking some time to go beyond learning about NPK elements. Sulfur and Boron jump out as two essential elements that gardeners need to know about.
- Sulfur: This is often overlooked, and it shouldn’t be! Sulfur is an anion, which means it’s negatively charged. This means that you need to actively add it back into the soil. Some crops need more sulfur than others. If a crop is a heavy feeder of nitrogen, you can assume it also needs a lot of sulfur.
- Boron: One of the most important micro-nutrients to watch, simply because it’s also often overlooked. Limited boron can lead to plant weakness.
Now it’s hopefully a little bit easier to dig into the chemical elements of your soil tests. It’s important to note that while soil chemistry is vital to understand, it’s only one component of your soil’s overall health. Another important aspect is soil biology, which relates to the number, size and makeup of the aggregates in your soil. These things you can learn by looking, touching, and noticing your soil. Then there’s soil physics as it relates to compaction levels in your soil. With these three sciences in mind, any of us can become experts in our own backyards.
Additional Resources for Understanding Basic Soil Chemistry
- http://www.ellenpolishuk.com/
- http://www.rodalesorganiclife.com/garden/soil-chemistry
- http://www.soils4teachers.org/chemistry
Next time on the blog we’re jump back into something practical: seed starting! It’s the time of year to get into the greenhouse (or kitchen, as it were) and get some seeds ready to sow for spring. I’ll take you through the basics and share my tips for success, so stay tuned.







 Family
Family

